Bremsstrahlung
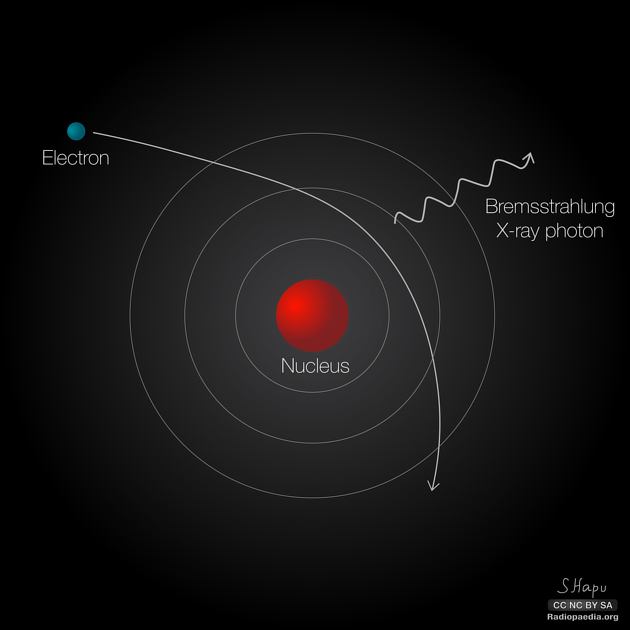
X-rays are produced by high-energy electrons bombarding a target, especially targets that have a high proton number (Z). When bombarding electrons penetrate into the target, some electrons travel close to the nucleus due to the attraction of its positive charge and are subsequently influenced by its electric field. The course of these electrons would be deflected, and a portion or all of their kinetic energy would be lost. The principle of the conservation of energy states that in producing the X-ray photon, the electron has lost some of its kinetic energy (KE):
- final KE of electron = initial KE of electron - energy of X-ray photon
The 'lost' energy is emitted as X-ray photons, specifically bremsstrahlung radiation (bremsstrahlung is German for 'braking radiation'). Bremsstrahlung can have any energy ranging from zero to the maximum KE of the bombarding electrons (i.e., 0 to Emax), depending on how much the electrons are influenced by the electric field, therefore forming a continuous spectrum. The 'peak' of the spectrum typically occurs at approximately one-third of Emax so for a bremsstrahlung spectra with an Emax value of say 120 keV, the peak of the spectrum would be at approximately 40 keV.
The intensity of bremsstrahlung radiation is proportional to the square of the atomic number of the target (Z), the number of unit charges of the bombarding particle (z) and inversely with the mass of the bombarding particle (m): Z² z / m. It follows that light particles such as electrons and positrons bombarding targets of high atomic number are more efficient producers of bremsstrahlung radiation than heavier particles such as alpha particles or neutrons (which can also cause X-rays to be produced through bremsstrahlung, though it's much more unlikely than with electrons).
Siehe auch:
und weiter:

 Assoziationen und Differentialdiagnosen zu Bremsstrahlung:
Assoziationen und Differentialdiagnosen zu Bremsstrahlung: