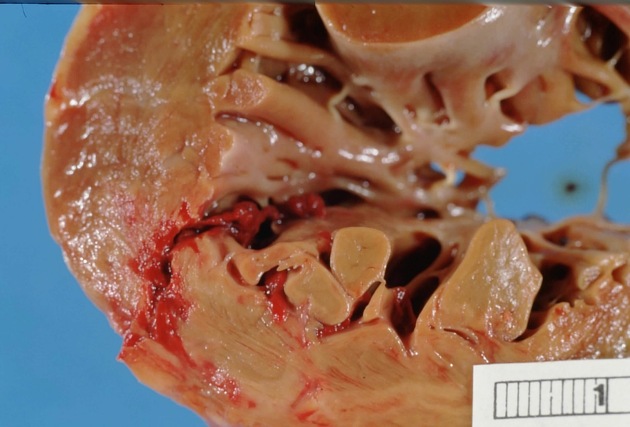
Hinterwandinfarkt

Hinterwandinfarkt
Myokardinfarkt Radiopaedia • CC-by-nc-sa 3.0 • de
Myocardial infarction (MI), an acute coronary syndrome, results from interruption of myocardial blood flow and resultant ischemia and is a leading cause of death worldwide.
Epidemiology
Risk factors
- male > females
- age
- >45 years for males
- >55 years for females
- cardiovascular risk factors: smoking, hypertension, LDL cholesterol, hyperlipidemia, diabetes, obesity, physical inactivity, air pollution
- positive family history: a history of first-degree male relative (i.e. brother, father, son) with MI <55 years of age or first-degree female relative (i.e. mother, sister, daughter) with MI <65 years of age
Clinical presentation
- chest pain/tightness, which may radiate down the left arm or into the jaw
- "silent" ischemia can occur in those with poor visceral sensation (diabetics, post-cardiothoracic surgery) and may manifest with other symptoms of myocardial compromise, e.g. breathlessness
Laboratory markers
The mainstay of diagnosis revolves around cardiac biomarkers: high-sensitivity troponin (and previously creatine kinase MB (CK-MB)) and electrocardiogram findings.
ECG
Findings on the ECG depending on the coronary artery involved. In a typical right dominant system, classic patterns include:
- proximal left anterior descending artery occlusion
- produces an "extensive anterior MI" pattern, with ST-segment elevation in precordial leads V1-6 and limb leads I and aVL
- reciprocal ST-segment depression in lead III
- mid-left anterior descending artery occlusion
- produces an "apical MI" pattern, with ST-segment elevation in precordial leads V3-6 and most of the limb leads
- elevation in lead II > III
- leads III and aVL both elevated (usually show reciprocity)
- reciprocal ST-segment depression in lead aVR
- produces an "apical MI" pattern, with ST-segment elevation in precordial leads V3-6 and most of the limb leads
- proximal right coronary artery occlusion
- usually produces an "inferior MI" pattern, with ST-segment elevation in limb leads II, III, and aVF
- reciprocal ST-segment depression in lead I and aVL
- associated right ventricular MI denoted by elevation of the ST segment in III>II and V1>V2
- associated posterior MI pattern has tall right (V1-3) precordial R waves with horizontal ST depression and tall, upright T waves
- left circumflex artery occlusion
- usually produces a "high lateral wall MI" pattern, with ST-segment elevation in limb leads I and aVL
- reciprocal ST-segment depression in lead III
Pathology
Coronary artery disease with rupture of an atherosclerotic plaque resulting in occlusion (local thrombosis/dissection) is the major cause of myocardial infarctions. Other causes include :
- ischemic imbalance (i.e. myocardial oxygen supply/demand imbalance)
- in critically-ill patients or in the setting of major (non-cardiac) surgery
- vasospasm
- iatrogenic, e.g. during revascularization procedures
Types
The most commonly used method of classification is as follows:
- type I: spontaneous MI related to ischemia from a primary coronary event (e.g., plaque rupture, thrombotic occlusion)
- type II: secondary to ischemia from a supply-and-demand mismatch (e.g. sepsis, hypotension, tachyarrhythmia, anemia)
- type III: MI resulting in sudden cardiac death
- type IV:
- type IVa: is an MI associated with percutaneous coronary intervention
- type IVb: associated with in-stent thrombosis
- type V: MI associated with coronary artery bypass surgery.
Location
The heart is supplied by three main coronary arteries. Thus, hypoperfusion patterns usually follow a territorial pattern:
- right coronary artery: supplies the thin (3 mm) walled right ventricle; this artery is dominant in 70% of patients, meaning that this artery supplies the inferior heart and posterior interventricular septum via the posterior descending artery
- left anterior descending artery: supplies the anterior part of the left ventricle and the anterior aspect of the interventricular septum
- circumflex artery: supplies the lateral and posterior aspect of the left ventricle; in 10% of patients, this artery is dominant, meaning that it supplies the inferior heart and posterior interventricular septum
For a more in-depth discussion of coronary dominance, see the article coronary arterial dominance.
Radiographic features
Secondary tests such as nuclear medicine (hot sestamibi) and echocardiography (localized hypokinesis) are used to aid in the diagnosis in some patients.
Given various advances in cardiac imaging such as:
- ECG gating
- dual source (effectively halving the rotation time of the tube)
- increasing detector area (256-row and 320-row single-source CT systems), allowing the entire heart to be scanned in 1 rotation (at significantly lower radiation doses - as low as 1 mSv in prospective ECG-triggered scanning)
CT scanning has the potential to play a central role in the investigation of chest pain. Apart from being able to detect large territory infarcts on coronary CT angiography (CTA), CT has the added advantage of being able to diagnose other causes of chest pain (e.g. pulmonary embolism, aortic dissection, pneumonia), in a protocol known as “triple rule-out” CTA.
Plain radiograph
Useful in excluding other causes of chest pain, e.g. pneumonia. Less useful in the direct diagnosis of myocardial infarction. The cardio-mediastinal contours are usually normal. One may occasionally see signs of heart failure.
CT
CT coronary angiogram
Most of the studies evaluating the usefulness of CT imaging have used 64 multislice CT scanning with ECG gating to assess the lumen of coronary arteries. Using this technique, a sensitivity of 92% and specificity of 76% was achieved, even in patients who were initially ECG and troponin negative .
"Triple rule-out” coronary CT angiography
Some institutions are using this protocol that examines for not only coronary artery disease, but also aortic dissection, pulmonary embolism, and other chest diseases. While there is a consensus about this protocol offering advantages in evaluating emergency department patients presenting with symptoms consistent with an acute coronary syndrome, there is an ongoing debate about proper indications. It should not be used routinely and lacks demonstration of increasing efficiency of resource use .
See triple-rule-out CT.
CT perfusion
In patients who have established coronary artery narrowing, CT perfusion can be used to predict the significance of the luminal narrowing as well as predicting post-infarction myocardial viability/salvageability .
An acute myocardial infarct would manifest with a reduced first-pass effect (hypodense myocardium). A CT thoracic aortogram is in effect a cardiac first-pass perfusion study (albeit, without the ECG gating) and has the potential to detect large territory myocardial infarcts. Despite these described findings, the role of CT perfusion in assessing acute myocardial infarction has not been well established.
An established myocardial infarct would manifest with:
- delayed enhancement (7-15 minutes post-CT contrast dose)
- delayed peak enhancement occurs slightly later compared to normal myocardium 12.8 versus 11.6 seconds
- peak enhancement is lowest in infarcts (26 HU) versus ischemia (36 HU) versus normal myocardium (58 HU)
Infarct scars can mimic acute myocardial infarcts as they demonstrate a similar enhancement pattern; however, old infarcts are often associated with myocardial thinning and contour abnormality (bulges away from ventricle), useful distinguishing features.
One study has assessed the utility of non-ECG-gated 16 slices CT pulmonary angiogram in detecting myocardial infarct. This method suffers from a few problems. Firstly, the relatively early (cf. with CT aortogram/coronary angiogram) phase results in non-homogeneous enhancement of the myocardium. Secondly, streak artefact (consider saline chaser) from the undiluted contrast in the SVC/right atrium caused "pseudo areas" of reduced myocardial attenuation. Thirdly, movement artefact from the beating heart caused areas of increased/decreased attenuation. Despite these problems, this study published optimistic figures of 66.6% (sensitivity) and 91.4% (specificity) .
Approaches using dual-energy CT to visualize late myocardial enhancement as a marker for scars showed only a limited diagnostic value in comparison to MRI .
Angiography
Digital subtraction angiography will show luminal arterial compromise. Primary percutaneous coronary intervention (primary PCI) with angioplasty and stenting is the gold standard for the treatment of ST-elevation myocardial infarct. Patients with non-ST-elevation myocardial infarction also commonly undergo coronary angiography as inpatients.
MRI
Recent advances in MRI have made it possible to assess myocardial infarction in patients with acute chest pain as well as those with subacute or chronic disease. Using different MR signals and techniques provides valuable information on assessing the scar tissue as well as salvageable myocardium.
In the acute phase of infarction, myocardial edema can be seen as T2 weighted high signal regions. It has been shown that these regions are salvageable. These segments of the myocardium are called "myocardium at risk".
Perfusion MRI at rest and during a vasodilator stress administration using a ‘first-pass' technique shows a signal increase in normal myocardium. Enhancement is limited in ischemic myocardium.
Myocardial scar tissue can be identified using late gadolinium enhancement (LGE) images and is useful in differentiating infarction (subendocardial or transmural) from non-infarcted myocardium or other non-ischemic cardiomyopathies and infiltrative diseases.
PET/MRI
In those who have had a myocardial infarct, PET/MRI can be used to identify patients with potentially viable/salvageable myocardium that may be a candidate for revascularization therapy (stunned myocardium or hibernating myocardium).
Echocardiography
The first manifestation of the ischemic cascade detectable by echocardiography is diastolic dysfunction, preceding derangement of systolic function. Other features suggestive of myocardial ischemia in an appropriate clinical context include:
- new regional wall motion abnormalities
- correspond to vascular territory affected
- adjacent segmental hyperkinesis
- previously infarcted myocardium will also demonstrate wall motion abnormalities, but is characteristically thin (<7 mm or <30% the thickness of adjacent myocardium) and displays increased relative echogenicity
- decreased systolic wall thickening
- normal wall thickening is >40%
- tardokinesis
- post-systolic thickening

 Assoziationen und Differentialdiagnosen zu Hinterwandinfarkt:
Assoziationen und Differentialdiagnosen zu Hinterwandinfarkt: