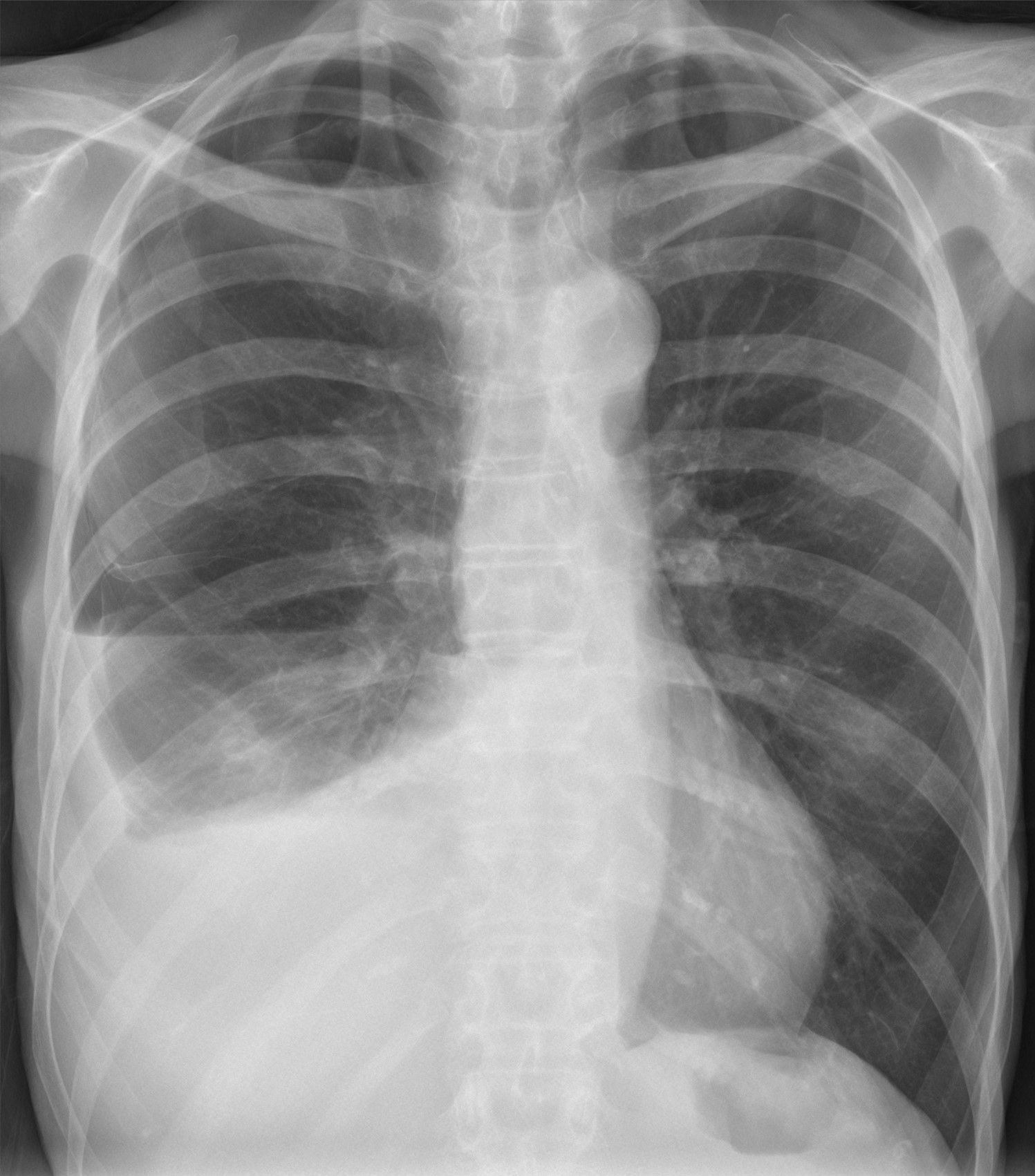
pleural effusion

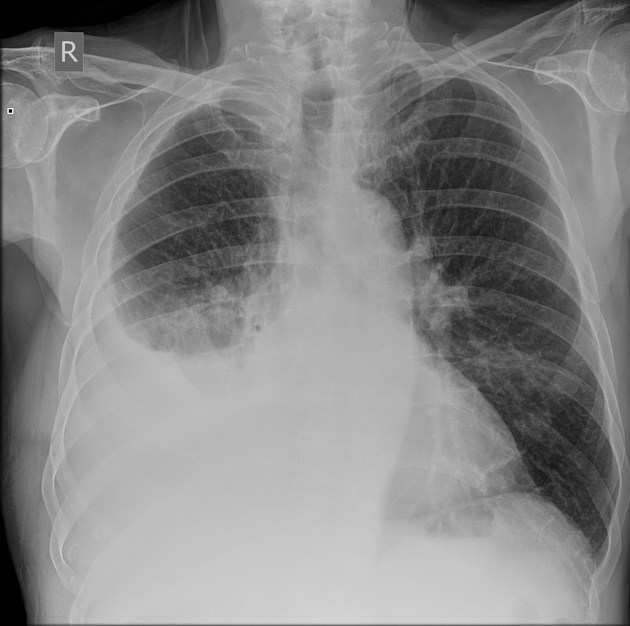
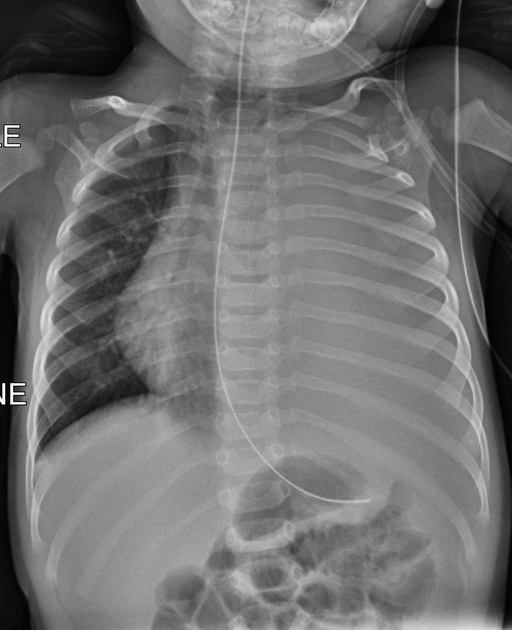
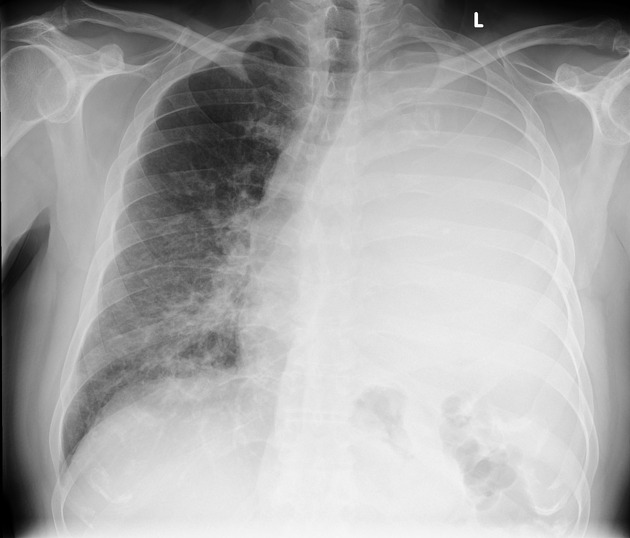
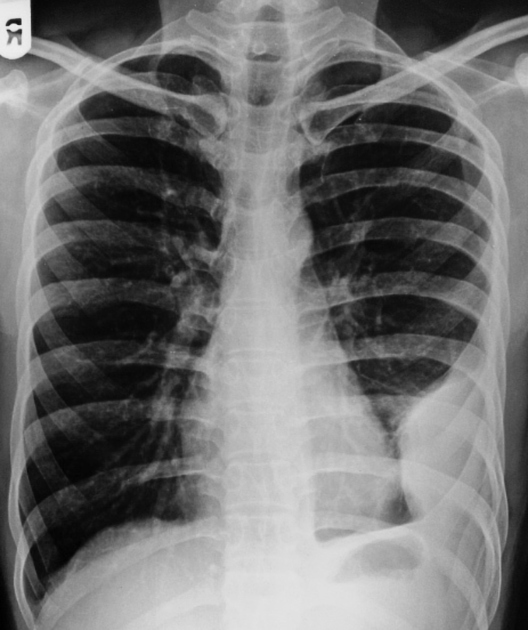
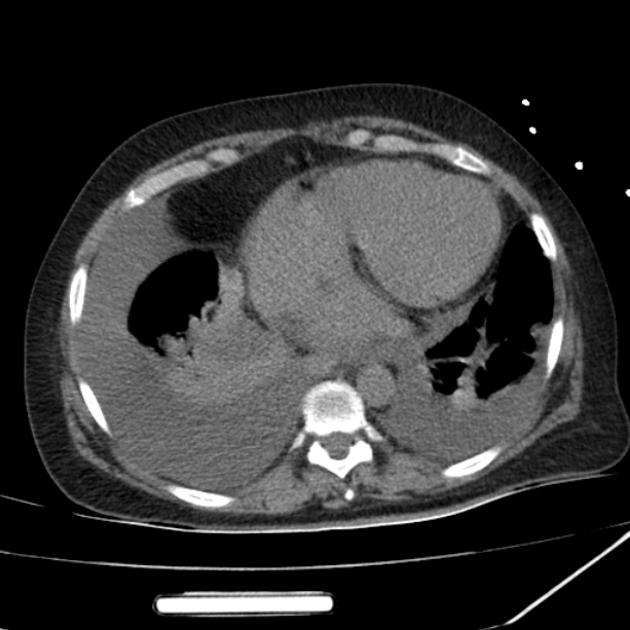
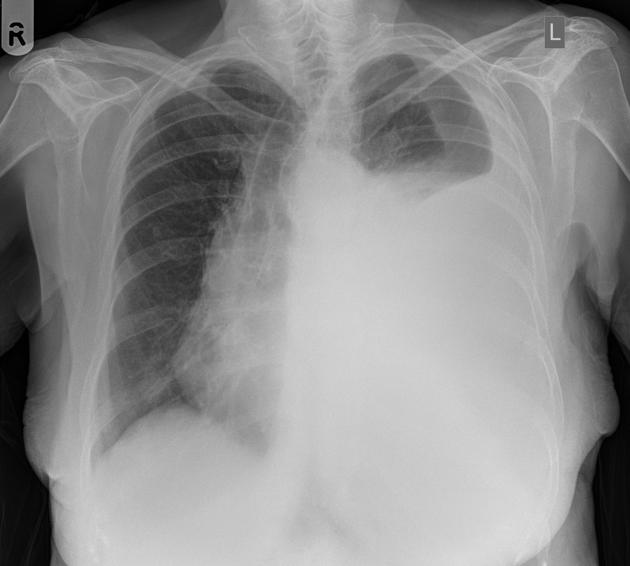


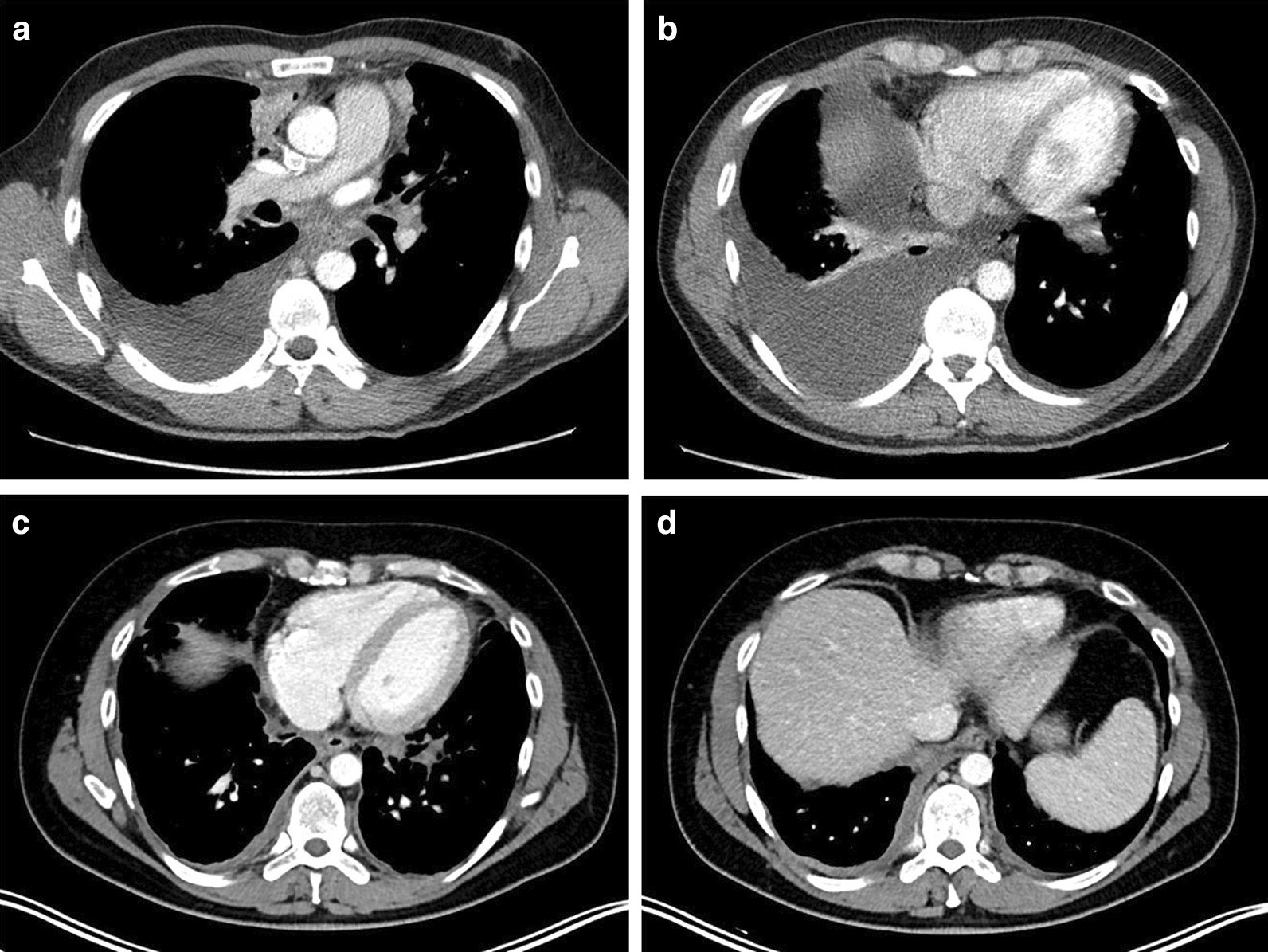
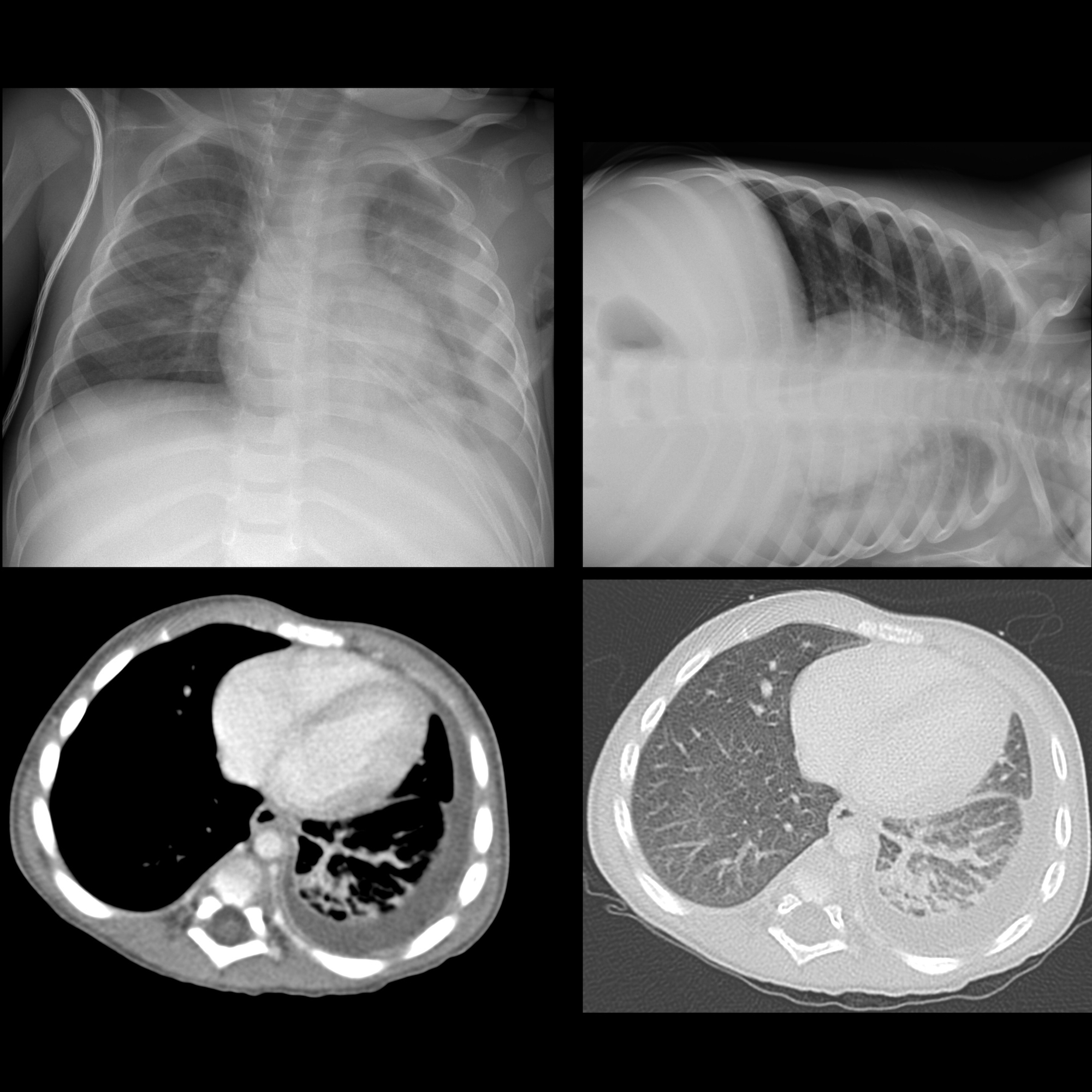



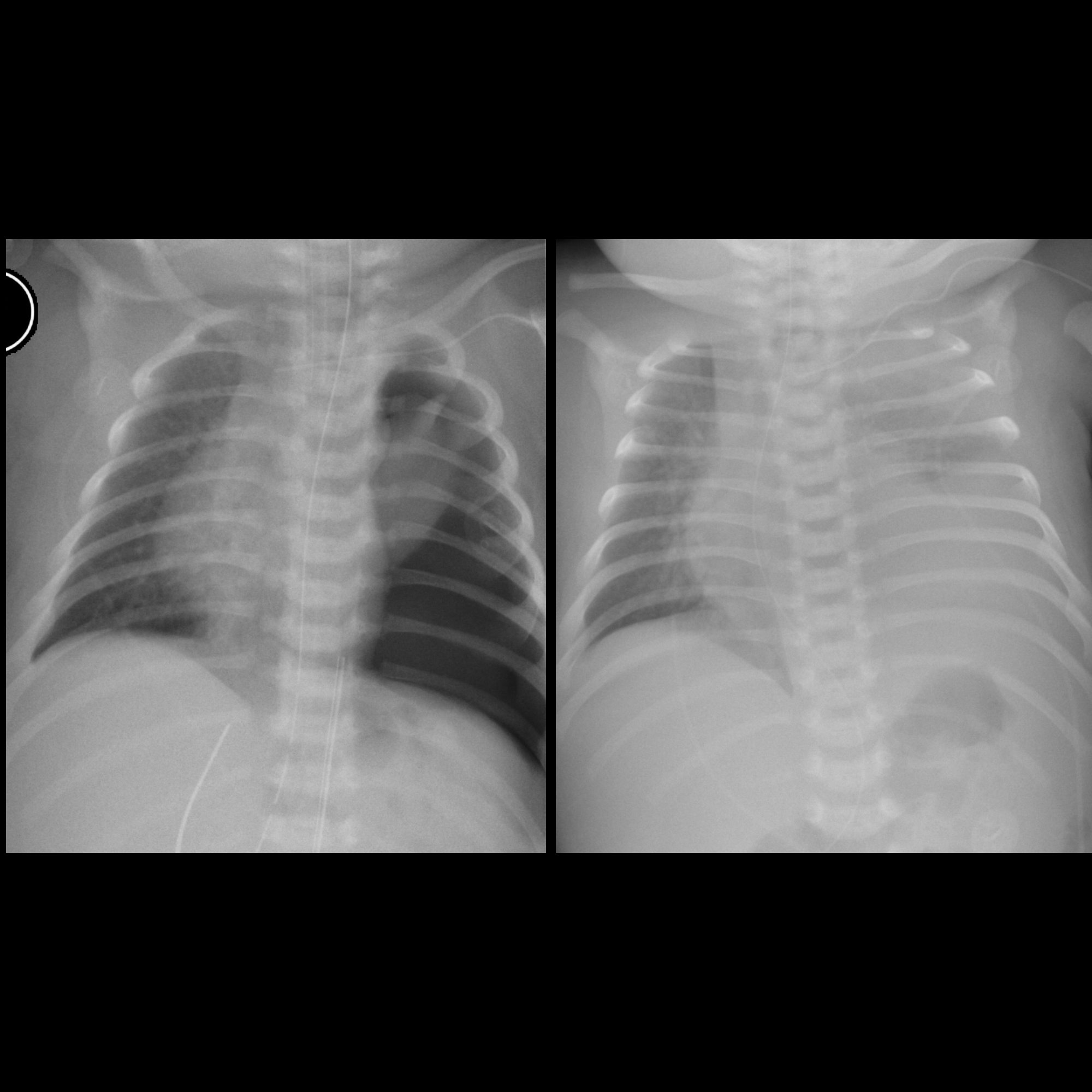
Pleural effusions are abnormal accumulations of fluid within the pleural space. They may result from a variety of pathological processes which overwhelm the pleura's ability to reabsorb fluid.
Terminology
"Pleural effusion" is commonly used as a catch-all term to describe any abnormal accumulation of fluid in the pleural cavity. The lack of specificity is mainly due to the limitations of the imaging modality. Given that most effusions are detected by x-ray, which generally cannot distinguish between fluid types, the fluid in question maybe simple (transudative) fluid, blood, pus, chylous fluid, etc.
If simple fluid, then the term hydrothorax may be employed, although this is rarely used (other than in combination terms e.g. hydropneumothorax)
If additional corroborative evidence is available, certain (mostly non-transudative) effusions are preferentially designated using more specific terminology. This is important because these effusions may be managed distinctly. These are discussed separately:
- chylothorax
- cholothorax
- glycinothorax
- hemothorax
- empyema (pyothorax)
- infusothorax (chemothorax)
- oleothorax
- pseudochylothorax
- urinothorax (urothorax)
Epidemiology
As the accumulation of fluid in the pleural space occurs in a broad range of disparate clinical scenarios, no single demographic is affected; rather the epidemiology will match that of the underlying condition. However, it is probably safe to say that as congestive cardiac failure and malignancy are some of the most common causes, older patients would be over-represented.
Clinical presentation
A small amount of fluid is completely asymptomatic. In fact, depending on the respiratory reserve of the patient, even large amounts of fluid can accumulate within the pleural space before any symptoms are recognized.
Eventually as the volume of fluid increases, with resulting passive (relaxation) atelectasis of the adjacent lung, the patient will experience reduced exercise tolerance and breathlessness.
Pathology
Physiologically, the pleural cavities normally contain approximately 15 mL of serous fluid . Any process which results in more fluid forming than can be absorbed will produce a pleural effusion.
Transudates vs exudates
There are many causes of pleural effusion that are broadly split into transudates and exudates. This categorization relies upon the biochemical analysis of aspirated pleural fluid :
- transudate
- protein concentration
- <30 g/L absolute
- total protein fluid:serum ratio <0.5
- lactate dehydrogenase (LDH)
- <20 IU/L
- LDH fluid:serum ratio <0.6
- specific gravity <1.016
- protein concentration
- exudate
- protein concentration
- >30 g/L
- total protein fluid:serum ratio >0.5
- lactic acid dehydrogenase (LDH)
- >20 IU/L
- LDH fluid:serum ratio >0.6
- specific gravity >1.016
- protein concentration
Exudate
It occurs due to the increase in permeability of the microcirculation or alteration in the pleural space drainage to lymph nodes. Examples:
- bronchial carcinoma
- secondary (metastatic) malignancy
- pulmonary embolism and infarction - pleural effusions in pulmonary embolism
- pneumonia
- tuberculosis
- mesothelioma
- rheumatoid arthritis
- systemic lupus erythematosus (SLE)
- lymphoma
Transudate
It occurs when there is an increase in hydrostatic pressure or a decrease of capillary oncotic pressure. Examples:
- cardiac failure
- nephrotic syndrome
- cirrhosis
- cirrhosis: hepatic hydrothorax
- Dressler syndrome
- trauma
- asbestos exposure
- yellow nail syndrome
- urinothorax
- post coronary artery bypass grafting: small unilateral left-sided pleural effusion can be common
- certain medications:
- dasatinib
Differential white cell count
Differential white cell count of the pleural aspirate is also important, resulting in pleural effusions with mainly granulocytes, eosinophils or lymphocytes .
Polymorphonuclear pleural effusion
Most pleural effusions with large numbers of polymorphs are acute :
- parapneumonic effusion: visible consolidation on chest radiograph
- pulmonary embolism (PE)
- tuberculosis
- asbestos associated pleural disease
Eosinophilic pleural effusion
Finding of an eosinophilic pleural effusion (>10% eosinophils) has no real clinical utility. They are usually found in the context of gas or blood in the pleural cavity, and do not exclude a malignant cause.
Lymphocytic pleural effusion
Most lymphocytic pleural effusions are due to :
- malignancy
- tuberculosis (maybe predominantly granulocytic)
less commonly:
- sarcoidosis
- rheumatoid lung disease
- chylothorax
Radiographic features
Plain radiograph
Chest radiographs are the most commonly used examination to assess for the presence of a pleural effusion; however, it should be noted that on a routine erect chest x-ray as much as 250-600 mL of fluid is required before it becomes evident . A lateral decubitus projection is most sensitive, able to identify even a small amount of fluid. At the other extreme, supine projections can mask large quantities of fluid.
Chest radiograph (lateral decubitus)
A lateral decubitus film (obtained with the patient lying on their side, effusion side down, with a cross table shoot through technique) can visualize small amounts of fluid layering against the dependent parietal pleura.
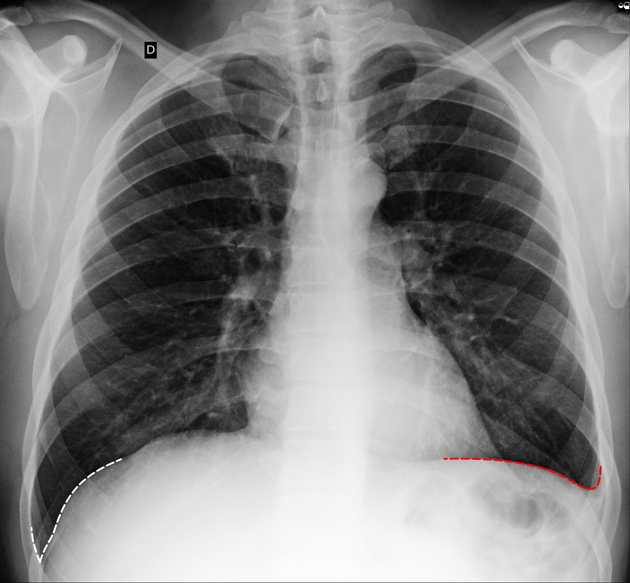
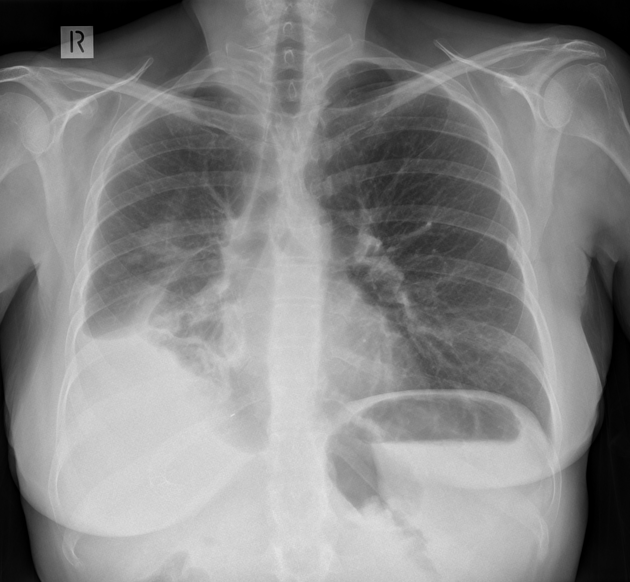
Chest radiograph (erect)
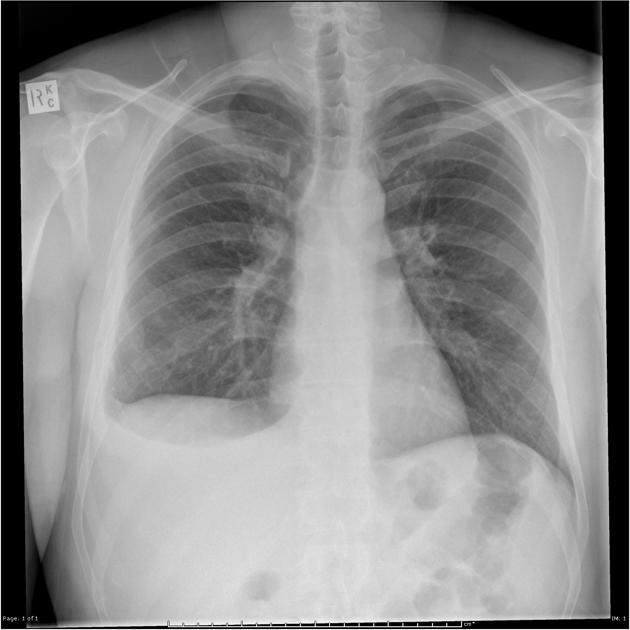
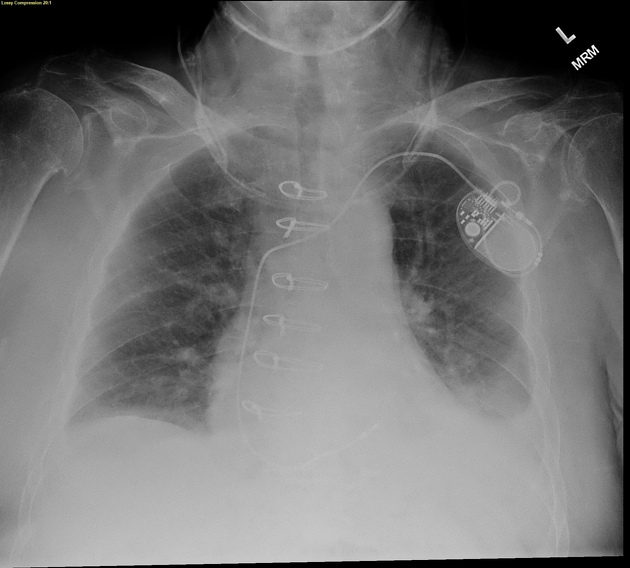
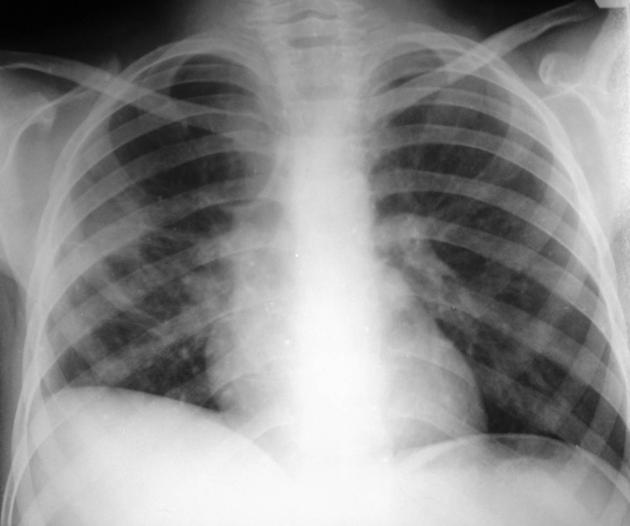
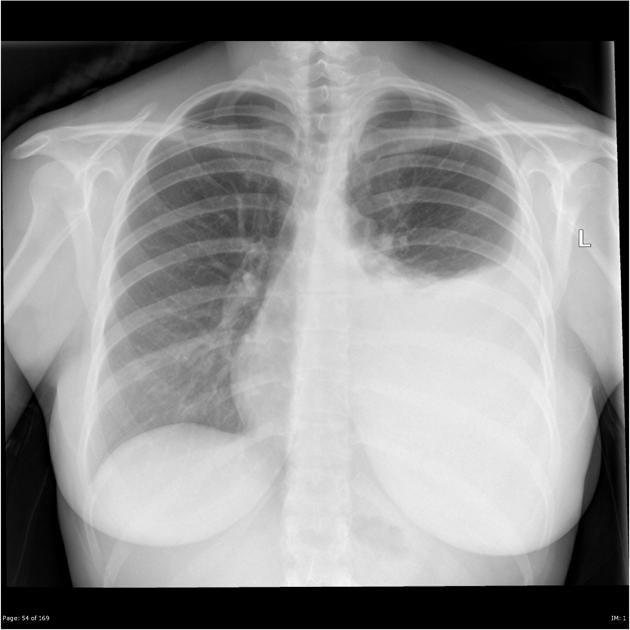
Both PA and AP erect films are insensitive to small amounts of fluid. Features include:
- blunting of the costophrenic angle
- blunting of the cardiophrenic angle
- fluid within the horizontal or oblique fissures
- eventually, a meniscus will be seen, on frontal films seen laterally and gently sloping medially (note: if a hydropneumothorax is present, no such meniscus will be visible)
- with large volume effusions, mediastinal shift occurs away from the effusion (note: if coexistent collapse dominates then mediastinal shift may occur towards the effusion)
Lateral films are able to identify a smaller amount of fluid as the costophrenic angles are deepest posteriorly.
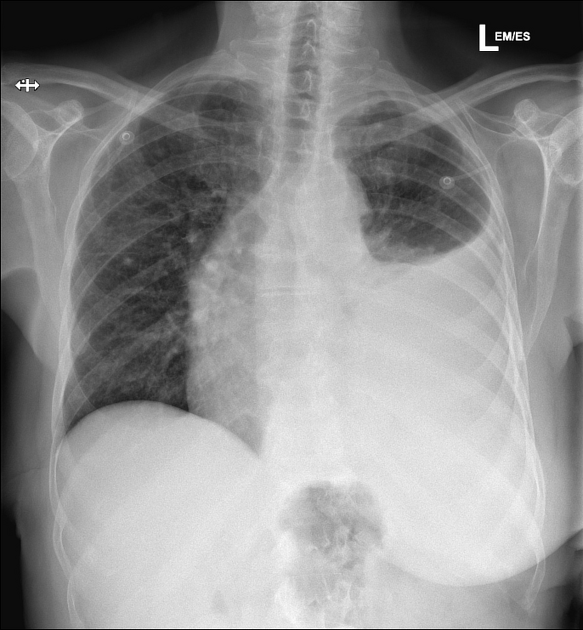
A subpulmonic effusion (a.k.a. infrapulmonary effusion) may be seen when there is previously established pulmonary disease, but can also be encountered in normal lungs. It can be difficult to identify on frontal radiographs. They are more common on the right, and usually unilateral. The following features are helpful :
- right: peak of the hemidiaphragm is shifted laterally
- left: increased distance between lower lobe air and gastric bubble
A lateral decubitus film is again ideal.
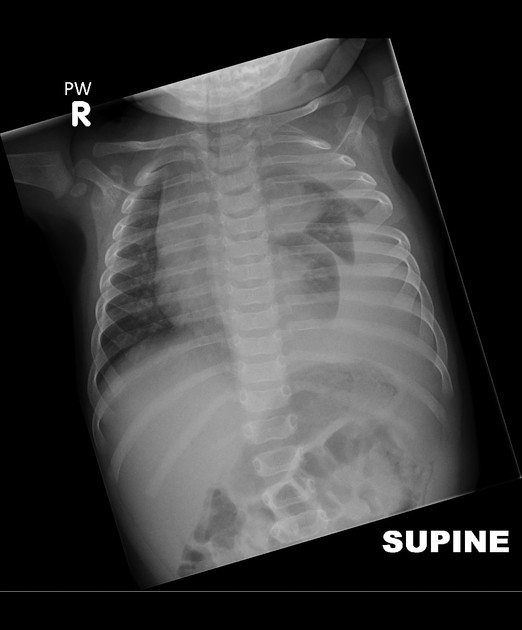
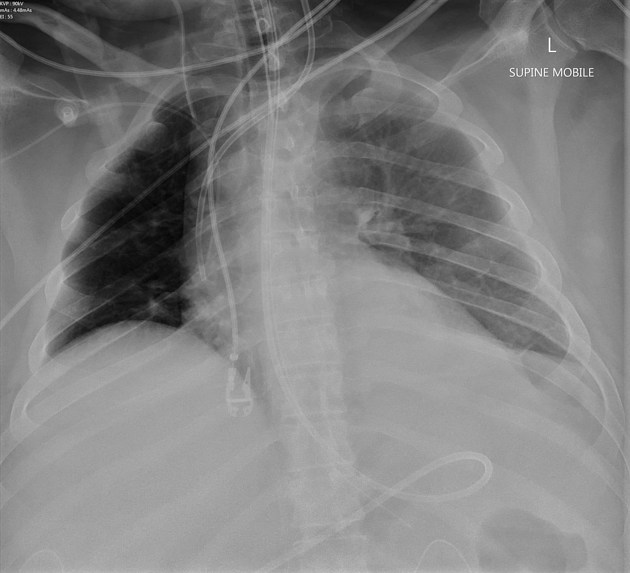
Chest radiograph (supine)
Large amounts of fluid can be present on supine films with minimal imaging changes, as the fluid is dependent and collects posteriorly. There is no meniscus, and only a veil-like increased density of the hemithorax may be visible. It is therefore especially difficult to identify similar sized bilateral effusions as the density of the lungs will be similar.
Ultrasound
Ultrasound allows the detection of small amounts of pleural locular fluid, with positive identification of amounts as small as 3-5 mL, that cannot be identified by radiographs, which is only capable of detecting volumes above 50 mL of liquid. Contrary to the radiological method, ultrasound allows an easy differentiation of loculated pleural fluid and thickened pleura. Moreover, it is effective in guiding thoracentesis (thoracocentesis), even in small fluid collections .
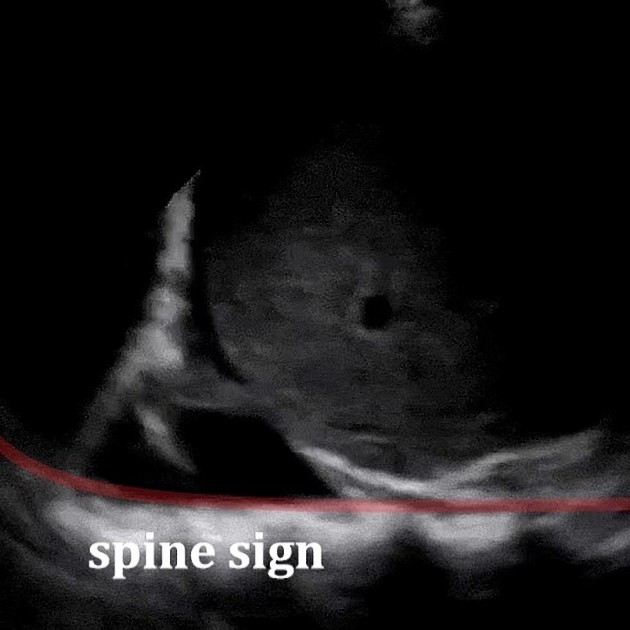
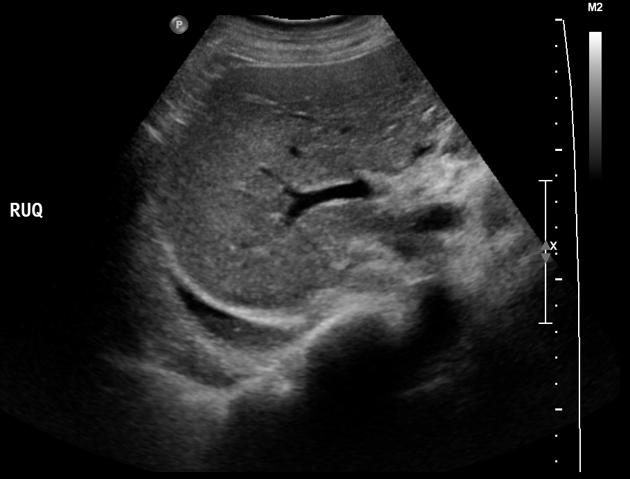
When viewed in a coronal plane, with the ultrasound transducer at the mid to posterior axillary line, the space above the hemidiaphragm is typically occupied by an artifactual reflection of hepatic (or splenic) architecture, with inspiratory obscuration of the (projected location of) the posterior costophrenic sulcus as the lung descends. The spine is also obscured as it extends into the thorax. One may observe how, with the collection of fluid superior to the hemidiaphragm :
- the space above the hemidiaphragm does not mirror the echogenicity of the liver
- the thoracic spine sign appears due to the excellent acoustic medium (fluid) interposed where air once was
- may be observed as a linear column of hyperechoic scallops with posterior acoustic shadowing extending beyond the diaphragm
- the defining sonographic features of effusion are the quad sign and sinusoid sign
- quad sign refers to the usual boundaries defining a pleural effusion
- two anechoic posterior rib shadows are the horizontal boundaries
- the parietal pleura and the visceral pleura are the remaining two surfaces, the latter being indistinguishable from the parenchyma it invests, usually referred to as the "lung line"
- an inspiratory decrease in the depth of the effusion, classically demonstrated in M-mode, is the sinusoid sign, a manifestation of intrinsically liquid dynamics (may be absent if extremely viscous)
- if underlying lung is submerged in a pleural effusion, when demonstrated in B-mode, the respiratory dynamics are reminiscent of a jellyfish, hence the jellyfish sign, a subtype of the sinusoid sign
- quad sign refers to the usual boundaries defining a pleural effusion
Homogeneously anechoic effusions may be either transudates or exudates, but any degree of heterogeneity is pathognomonic of a complex effusion (non-transudative). Exudative effusions often demonstrate punctate, hyperechoic foci floating within the effusion, referred to as the plankton sign. Septations may be seen in the pleural fluid, and may indicate underlying infection but can be seen in chylothorax or hemothorax . The appearance of the "hematocrit sign" may be observed in hemothorax, with a surface layer of anechoic fluid sitting atop a settled, fine echogenic sediment.
Ultrasound can be used in the assessment of pleural effusion volume. Refer to the article "Pleural effusion volume (ultrasound)" for more information
CT
CT scanning is excellent at detecting small amounts of fluid and is also often able to identify the underlying intrathoracic causes (e.g. malignant pleural deposits or primary lung neoplasms) as well as subdiaphragmatic diseases (e.g. subdiaphragmatic abscess).
CT is not able to differentiate between a transudative or exudative pleural effusion with similar fluid densities and non-differentiating rates of loculation and pleural thickening . However, CT can help distinguish between a pleural effusion and a pleural empyema (see pleural effusion vs pleural empyema).
Treatment and prognosis
The treatment of pleural effusions is usually targeted to the underlying condition (e.g. congestive cardiac failure or malignancy). Symptomatic patients with large effusions may be treated by therapeutic aspiration (thoracentesis).
When effusions are very large, this can safely be done 'blind' although increasingly ultrasound is used to at least mark an appropriate site. Ultrasound-guided aspiration is reliable and fast and enables loculated effusions to be drained. A catheter can be left in situ, although care must be taken to ensure that it is connected either to an underwater drain or to a sealed system such that air cannot enter the pleural cavity.
If effusions reaccumulate despite repeated aspirations and systemic therapy (where appropriate), a tunnelled semipermanent pleural drain or video-assisted thoracic surgery (VATS) pleurodesis can be considered.
Differential diagnosis
Imaging differential considerations include:
- raised hemidiaphragm, e.g. hepatomegaly, phrenic nerve palsy
- collapse or consolidation
- pleural thickening, e.g. old tuberculosis or empyema
- inferior pulmonary ligament
- poor radiographic technique
Practical points
- strange or atypical configurations of pleural fluid can be due to either adhesions (i.e. loculated effusion) or underlying atelectasis. The latter are more likely to change with patient positioning
See also

 Assoziationen und Differentialdiagnosen zu Serothorax:
Assoziationen und Differentialdiagnosen zu Serothorax: